Chemistry is your friend
Who out there loves chemistry? We depend on chemistry to keep our water safe for drinking. Read on to learn about one of the most important chemistry mechanisms - disinfection.
We use lots of chemistry for water and wastewater treatment. Here, I’ll talk about one of the big ways in which chemistry is our friend and helps keep our drinking water safe - disinfection.
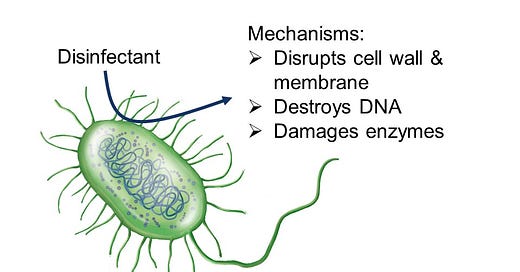
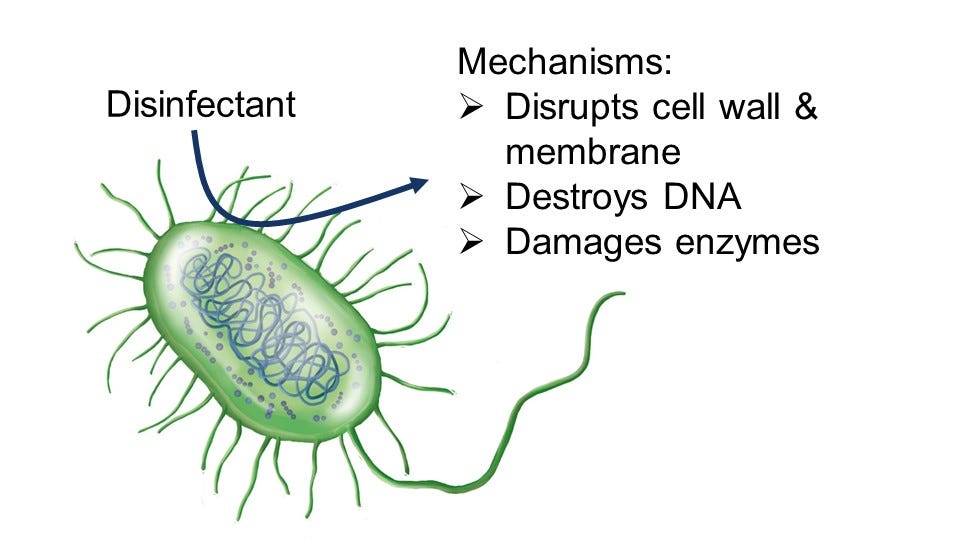
You know that disinfection essentially means we are doing something to the water to kill any living organism that might be harmful. But how does it really work?
Disinfectants include chemicals that are reactive in water or other sources of energy that cause reactions to happen in water. For disinfectants to work well at killing microbes, the reactions that occur must involve organic compounds, because all living organisms are made up of organic compounds. What is an organic compound? The simplest definition is any compound, or molecule, that contains the element carbon.
So humans and microbes and all other organisms contain many types of organic compounds, in that we are all made up of similar types of molecules, including amino acids, lipids (i.e., fats), nucleic acids in DNA, sugars, and others.
Disinfectants essentially are really good at reacting with these organic molecules, breaking them down into smaller compounds. In the process of reacting with organic compounds, structures that make up a microbe get destroyed - these can include the cell wall, the cell membrane, DNA, and enzymes. Once these structures start to be destroyed, microbes are unable to survive - and disinfection is achieved.
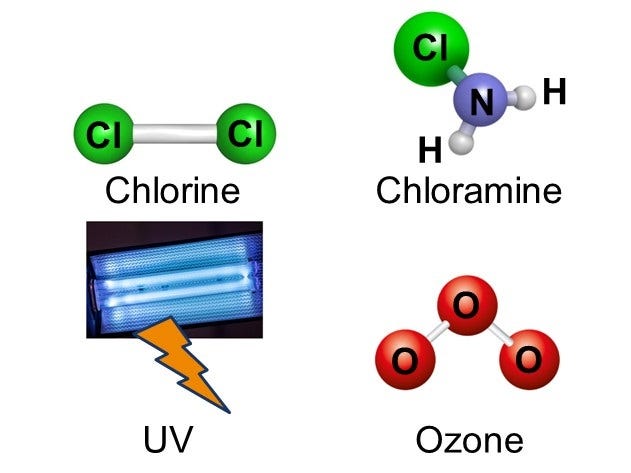
There are several primary types of chemical disinfectants that we use in drinking water treatment and wastewater treatment. One of the most-used disinfectant chemicals is chlorine. Chlorine is a simple molecule comprised of two chloride atoms covalently bonded together and denoted as Cl2. What is a covalent bond? A covalent bond is essentially two atoms sharing electrons together so strongly that the atoms stay close together in a compound, rather than separating.
Two other common disinfectant chemicals are chloramine and ozone. Chloramine is produced by combining chlorine and ammonia, while ozone is created by passing oxygen through UV radiation. Today, we have technologies that enable us to produce both chloramine and ozone easily and safely for use in water treatment.
Finally, UV can be used directly on water to disinfect - both ozone and UV systems are more often used in wastewater treatment, rather than drinking water treatment systems. More on the differences between drinking water and wastewater treatment to come in future posts.
There are advantages and disadvantages of these different disinfectants, and I’ll get into more details on that in future posts. Each of these disinfectants has different lifetimes in which they are effective, limitations on how and when they can and should be used, costs, and safety considerations.
One of the primary downsides to disinfection is what is known as disinfection by-products, or DBPs. DBPs are compounds that are produced as a result of using a disinfectant, and the types of compounds we include have primarily negative impacts on human health. Remember, disinfectants react with organic compounds, and this includes not only microbes but also any other organic compounds that are in the water. DBPs have been found to be carcinogenic, and the water treatment industry has figured out how to optimize disinfection treatment so that DBP production is minimized but disinfection is still achieved. DBPs can also be removed after a disinfection step and prior to the water being sent to people’s homes. Remember, there are always trade-offs and pretty much never a silver bullet perfect solution. We’ve learned this lesson with DBPs over the past several decades and now know how to control them.
Stay tuned in future weeks for more on chemistry - there are so many ways that chemistry is fundamental to us being able to produce safe drinking water and treat our wastewater.